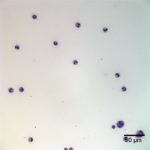
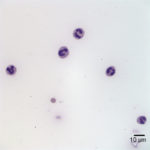
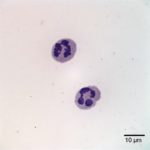
Interpretation
Marked septic suppurative inflammation.
Explanation
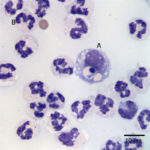
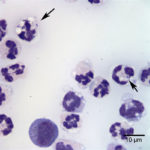
Examination of the direct smears prepared from the submitted fluid were highly cellular. The smears consisted of 97% neutrophils (Question 1, cell labeled as “B”) and 3% macrophages (Question 1, cell labeled as “A”). There were rare lymphocytes and eosinophils, and occasional clusters of reactive mesothelial cells on a background of a small amount of blood. The neutrophils were mostly non-degenerate with low numbers of mildly degenerate forms also present. There were low numbers of apoptotic neutrophils. Rarely, the neutrophils contained phagocytized pleomorphic rods (Question 2, Figure 4). Macrophages were often vacuolated and occasionally leukophagocytic of neutrophils (Figure 3).
The total protein concentration (>2.5 g/dL), total nucleated cell count (>10.0 thousand/µL), and increased proportion of neutrophils (>80%) were consistent with an exudate (Question 2) with intracellular bacteria indicating a septic process.
Additional information
A Gram stain of the peritoneal fluid revealed the bacteria to be Gram-negative rods. Given the cytologic results, an abdominal ultrasound and bacterial culture and antibiotic sensitivity testing of the peritoneal fluid were performed. The abdominal ultrasound revealed no abnormalities, whereas the bacterial culture yielded Actinobacillus spp.
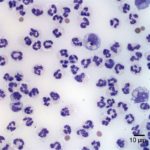
The horse was initially treated with penicillin G potassium, and after sensitivity results, the horse was also started on enrofloxacin (3.9 g intravenously every 24 hours). The horse continued to improve and 4 days after initial presentation, a recheck abdominal ultrasound and abdominocentesis were performed. The abdominal ultrasound revealed no abnormalities. The peritoneal fluid was medium-yellow in color and slightly cloudy. The total protein concentration by refractometry was <2.5 g/dL and the total nucleated cell count was 31.0 thousand/µL with 6.2 thousand red blood cells/µL. Most of the cells were non-degenerate neutrophils (94%, Figure 5-7) with a few macrophages (5%), and low numbers of lymphocytes (1%). Bacteria were not seen.
The horse remained comfortable, showed good appetite, and was bright, alert, and responsive the day after repeat abdominocentesis. After a final dose of intravenous antibiotics the horse was discharged to the owner’s care.
Based on cytologic and bacterial culture results and clinical progression of the horse, the final diagnosis was primary peritonitis due to Actinobacillus spp.
Discussion
Actinobacillus spp. are Gram-negative pleomorphic bacteria rods that, although part of the normal flora of the equine gastrointestinal tract, are known to cause disease in foals and adult horses. The most commonly isolated species are A. equuli subsp. equuli and A. equuli subsp. haemolyticus. Actinobacillus equuli subsp. equuli (A. equuli) is a common cause of septicemia in foals (sleepy foal disease) leading to characteristic lesions in multiple organs (e.g. multifocal embolic nephritis and pneumonia).1 Systemic infections in adult horses by A. equuli are considered rare;2 with infections being most often localized to specific organ systems or body cavities. Cases of abortion, pleuropneumonia, arthritis, pericarditis, periorchitis, enteritis, and peritonitis have been reported.1
Primary peritonitis due to A. equuli is uncommon, with a few reported cases in Australia3,4,5 and New Zealand, and fewer reports in North America.2,6,7 Most cases have been reported in adult horses, but horses as young as 9 months old may be affected.3 Reported cases of horses with Actinobacillus peritonitis describe presenting signs of lethargy, depression, decreased appetite, and mild to moderate abdominal pain (colic). Most horses are tachycardic, tachypneic and febrile. Decreased borborygmi are also commonly reported and rectal palpation is usually unremarkable.3,5,7 In some cases, firm, dry feces may be palpated in the rectum or large intestine.3 Although the findings from rectal palpation in this horse were not typical for primary peritonitis, they quickly resolved without requiring surgical intervention.
Similar to the peritoneal fluid from this horse, analysis of peritoneal fluid typically reveals results consistent with a peritonitis. The fluid tends to be turbid, abnormally-colored (creamy yellow to orange), and opaque. Total protein by refractometry is high, with an approximate range of 2.5-8.4 mg/dL. The nucleated cell count tends to be quite high (>100.0 thousand/µL in most cases), with the majority of the cells being neutrophils (up to 98% of nucleated cells). Neutrophils may be non-degenerate to less often mildly degenerate. Less numerous are macrophages (which may be leukophagocytic) and lymphocytes. Extracellular and/or intracellular Actinobacillus rods may or may not be seen.3,5,7 Similar to the horse in this case, a rapid and considerable decrease (>90% decrease) in the nucleated cell count after initiation of antibiotic therapy is typically reported. 3,5,7
Unlike with other causes of septic peritonitis, horses with A. equuli peritonitis usually do not progress to endotoxic shock, die or are euthanized. Most horses show a rapid response to antibiotic treatment within the first 48 hours without the need to perform a peritoneal lavage. The prognosis is considered good to excellent (Question 3).3,5 The horse in this case likewise showed a rapid improvement after initiation of therapy and was discharged 5 days after admission.
In this horse, primary peritonitis due to A. equuli was considered as the leading differential due to the absence of ultrasonographic abnormalities (such as abdominal masses), lack of a history of recent abdominal surgery or substantial hematologic abnormalities, and the rapid response to treatment. The positive bacterial culture confirmed this diagnosis. The mechanism by which A. equuli accesses the peritoneal cavity remains speculative. It has been proposed that strongyle intestinal migration may facilitate the translocation of bacteria from the intestine into the peritoneal cavity.8 Data from some reports question this association, however, and proposed that intestinal migration of foreign bodies may also be involved.5,7 Fecal flotation and fecal egg count were not done in this horse and no foreign bodies were identified on rectal palpation or abdominal ultrasound. Therefore, the potential inciting cause for the Actinobacillus peritonitis in this case could not be determined.
Although uncommon, Actinobacillus equuli, should be considered as a differential diagnosis in horses with septic peritonitis, particularly those without clinical signs of concurrent endotoxemia. The prognosis is considered good because of the usual rapid response to treatment without the need of surgical intervention, compared to other forms or causes of septic peritonitis (e.g. ruptured intestinal tract, strangulation).
References
- Layman QD, Rezabek GB, Ramachandran A, Love BC, Confer AW. A retrospective study of equine actinobacillosis cases: 1999-2011. J Vet Diagn Invest [Internet]. 2014;26(3):365–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24742921.
- Patterson-Kane JC, Donahue JM, Harrison LR. Septicemia and peritonitis due to Actinobacillus equuli infection in an adult horse. Vet Pathol. 2001;232(2001):230–2.
- Matthews S, Dart AJ, Dowling BA, Hodgson JL, Hodgson DR. Peritonitis associated with Actinobacillus equuli in horses: 51 cases. Aust Vet J [Internet]. 2001;79(8):536–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11599812.
- Gay CC, Lording PM. Peritonitis in horses associated with Actinobacillus equuli. Aust Vet J. 1980;56(6):296–300.
- Mogg TD, Dykgraaf S. Actinobacillus Peritonitis in a Warmblood Gelding. Vet Clin North Am – Equine Pract. 2006;22(1):9–16.
- Schneider RK, Meyer DJ, Embertson RM, Gentile DG, Buergelt CD. Response of pony peritoneum to four peritoneal lavage solutions. Am J Vet Res. 1988;49(6):889–94.
- Watts A, Johnson A, Felippe M, Divers T. Recurrent Actinobacillus peritonitis in an otherwise healthy Thoroughbred horse. Aust Vet J. 2011;89(4):143–6.
- Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J [Internet]. 2000;159(1):18–36. Available from: http://www.sciencedirect.com/science?_ob=ShoppingCartURL&_method=add&_eid=1-s2.0-S1090023399904033&originContentFamily=serial&_origin=article&_ts=1475362657&md5=6eabf9e318dd6f6925d1701d4086cb3f
Authored by: Dr. D. Hernandez , with minor edits by Dr. T. Stokol