Fluid cannot normally be aspirated from the abdomen in small animals (dogs, cats) but small amounts can be collected from the abdomen of large animals (horses, ruminants, camelids). Thus, interpretation of peritoneal fluid results includes the concept of “normal values” for the latter species, whereas any abdominal fluid that has accumulated is abnormal in small animals (i.e. there are no normals). Regardless of species, the same mechanisms of fluid accumulation apply, i.e. transudation, vessel/viscus rupture/leakage, exudation and neoplasia.
Transudative effusions
A transudative effusion develops in the abdominal cavity due to changes in plasma hydrodynamic pressure (e.g. venous hypertension) or changes in plasma oncotic pressure (i.e. hypoalbuminemia). Both mechanisms may be operative in a single patient. Abdominal fluid in small animals is classified into low (protein-poor) and high protein (protein-rich) transudates, which helps determine the mechanism of the effusion and potentially the underlying cause. Chronically increased hydrodynamic forces may cause endothelial perturbations, including altered integrity of interendothelial junctions and incitement of a mild inflammatory response (Sato and Ohashi 2005, Sidall et al 2017, Prystopiuk et al 2018), which may explain protein-rich transudative effusions as well as high neutrophil percentages in these effusions.
Low protein transudates (also called “pure” transudates)
These are usually caused by presinusoidal hypertension, i.e. hypertension originating between the portal triad in the liver and the intestinal tract. This is because lymphatic fluid draining from the intestine into the sinusoids of the liver is low in protein. A severe hypoalbuminemia (< 1.0-1.5 g/dL) can also result in a low protein transudate but hypoalbuminemia by itself is insufficient to cause substantial transudation. This is because globulins will also exert oncotic pressure and compensatory increases in lymphatic drainage will reduce fluid accumulation. Therefore, there are usually concurrent changes in hydrodynamic pressure (e.g. portal hypertension) leading to the effusion in animals with hypoalbuminemia (i.e. more than one mechanism is operative).
The cytologic characteristics of a low protein transudate in small animals are:
- Nucleated cell count: < 1,500/uL (pure transudate) or <5,000/uL (protein poor transudate)
- Red blood cell (RBC) count: Variable, but usually < 5,000/ul, unless there is blood contamination.
- Total protein (by refractometer): <2.5 g/dL
- Cytologic features: Mixture of non-degenerate neutrophils and macrophages (in one study of 63 dogs, there was a median and range of 35%, 0-100% neutrophils in fluids classified as low protein transudates based on nucleated cell count <1,5000/μL and total protein <2.0 g/dL [Alonso et al 2021a]), with low numbers of small lymphocytes and mesothelial cells. Reactive lymphocytes or plasma cells may be seen. Usually, there is minimal erythrophagia (indicating hemorrhage or extravasation of RBC into the abdominal cavity) in a freshly collected and prepared sample.
Some causes of a low protein transudate are:
- Portal (presinusoidal) hypertension from liver disease: This is the most common cause in both dogs and cats, e.g. hepatic cirrhosis, portosystemic shunts or vascular anomalies.
- Protein-losing nephropathy: The fluid resembles “water” in these conditions. In protein-losing nephropathy, glomerular disease leads to the loss of large amounts of albumin in the urine (decreased plasma oncotic pressure) and sodium retention within the kidney (increased plasma hydrodynamic pressure).
- Acute uroabdomen: This often has a high proportion of neutrophils (65-100% with a median of 95% in one study of 20 dogs [Alonso et al 2021a]).
High protein transudates
High protein transudates develop due to increased plasma hydrodynamic pressure (usually because of venous congestion) in the liver where hepatic sinusoids are more permeable to protein and indicate sinusoidal or post-sinusoidal hypertension (i.e. pressure originating within the liver or proximal to the central vein of the liver). These transudates used to be called “modified” transudates, but this term is falling out of favor.
The cytologic characteristics of a high protein transudate in small animals are:
- Nucleated cell count: < 5,000/uL
- Red blood cell (RBC) count: Variable, but usually <50,000/ul
- Total protein (by refractometer): >2.5 g/dL (and usually < 5.0 g/dL)
- Cytologic features: Mixture of non-degenerate neutrophils and macrophages (roughly 50% neutrophils; in one study of 84 dogs, the median and range of neutrophil percentages was 59%, 0-100% in protein rich transudates identified on the basis of nucleated cell counts ≤5000/μL and total protein ≥2.0 g/dL [Alonso et al 2021a]), with low numbers of small lymphocytes and mesothelial cells. Reactive lymphocytes, plasma cells or mesothelial cells may be seen. Usually, there is some erythrophagia and hemosiderophages (indicating hemorrhage or extravasation of RBC into the abdominal cavity). Thus, cytologic features are similar to a low protein transudate, although the RBC count is frequently higher with evidence of erythrophagia. Eosinophil counts are higher in these effusions versus other types of effusions in one study (Alonso et al 2021a).
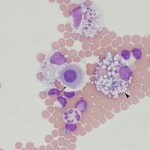
Some causes of a high protein transudate are:
- Post-sinusoidal hypertension
- Congestive heart failure in dogs: Decreased cardiac output can lead to venous congestion within multiple organs, including liver and lungs.
- Caudal vena cava thrombosis
- Neoplasia: Exfoliating or non-exfoliating
- Sinusoidal hypertension
- Liver disease: Diseases within the liver affecting the sinusoids or central vein may cause leakage of high protein fluid into the abdomen, e.g. chronic active hepatitis.
- Other causes of increased venous pressure: Neoplasia and torsion of abdominal organs.
Note that there may be an inflammatory component, with increased vascular permeability without chemotaxis, in effusions that are primarily caused by transudative mechanisms. This can be seen in animals with serosal inflammation or capillary leak syndrome (Sidall et al 2017). The latter is suspected in cases with nucleated cell counts towards the upper limit (3-5000/uL) in small animals or in the equivocal range in horses (5-10,000/uL), with a high proportion of neutrophils (>60-70%) and perhaps evidence of cell death (large individualized apoptotic cells) or other abnormalities (excessive leukophagia, necrotic cell debris within macrophages).
Lymphocyte-rich effusions
Lymphocyte-rich effusions are usually variants of a transudative effusion and due to leakage of lymph into the abdominal cavity. Since lymph draining from the intestine into the thoracic duct contains chylomicrons (absorbed from the intestine after eating), these effusions are frequently white or pink and opaque in appearance because they are rich in this large lipoprotein (pink is usually from concurrent hemorrhage or blood contamination). Thus, they are often called chylous effusions. Small lymphocytes dominate in these fluids. Unlike thoracic chylous effusions into the thorax, chylous ascites is relatively uncommon. In some patients, the fluid can be lymphocyte-rich but lacking chyle. This could be due to inappetence or anorexia in affected patients or due to leakage of lymph that is not specifically draining the intestinal tract. We have also seen an intestinal small cell lymphoma produce a (neoplastic) small lymphocyte-rich effusion, likely due to concurrent lymphangiectasia or lymphatic obstruction but this is quite rare.

The cytologic characteristics of a chylous effusion in small animals are:
- Gross appearance: Turbid, opalescent, may form a fat layer with storage (particularly refrigerated) from chylomicrons being less dense. Opacity does not precipitate on centrifugation (supernatant is still lipemic).
- Nucleated cell count: Variable, but usually >3,000/uL
- Red blood cell (RBC) count: Variable.
- Total protein (by refractometer): Variable. May be inaccurate due to lipemia interference with measurement.
- Cytologic features: High proportion of small lymphocytes is characteristic, along with other cavity fluid cells (non-degenerate neutrophils, macrophages). Both macrophages and neutrophils may have clear discrete-margined cytoplasmic vacuoles, indicating lipid ingestion. May see low numbers of large lymphocytes and plasma cells or reactive lymphocytes. Chyle is irritating so concurrent neutrophilic or mixed neutrophilic histiocytic inflammation is possible and may even dominate, resulting in nucleated cell counts >5,000/uL. We have also seen a grossly chylous abdominal effusion in horses with pancreatitis (uncommon).
- Confirmation: To confirm a chylous effusion, triglycerides could be measured in the fluid and serum and will be higher in the fluid (usually greater than 2x that of serum). Chylous effusion can also be confirmed if there is a cholesterol:triglyceride ratio in the fluid of <1. In reality, the vast majority of chylous effusions have triglyceride concentrations > 100 mg/dL so it may not be necessary to measure triglyceride concentrations in serum as well. At Cornell University, we rarely receive requests to run cholesterol on fluid, because pseudochylous effusions (due to increased cholesterol) are quite rare in animals. Note, the triglyceride concentration (or ratio with serum) may not be helpful for confirming the diagnosis in animals that have not been eating (fluid from anorectic animals may also not be grossly chylous).
Causes of a chylous ascites are uncommon and include:
- Lymphatic obstruction, e.g. tumor in mesenteric lymph node, mesenteric torsion, adhesions.
- Lymphangiectasia, e.g. intestinal disease, e.g. lymphoma.
- Pancreatitis (horses)
Exudative effusions
Exudates develop when inflammatory mediators cause increased vascular permeability that allows exudation of proteins and extravasation of inflammatory cells (most commonly neutrophils). These mediators also cause vasodilation, which can exacerbate the effusion by increasing blood flow to the capillaries in the area. Exudates can be caused by infectious (bacterial, viral, fungal, etc.) or non-infectious (foreign body, necrosis with a tumor, uroperitoneum, bile) causes. In some cases, exudation can occur in the absence of chemotaxis, resulting in high protein but normal cell counts. This is typical of feline infectious peritonitis, where vasculitis and production of vasoactive mediators by infected monocytes contributes to an exudative effusion without substantial neutrophil chemotaxis. For instance, Takano et al (2011) showed that FIP infection in monocytes or alveolar macrophges induces mRNA production and secretion of vascular endothelial growth factor compared to mock-infected cells and virus-infected monocyte culture supernatant increased permeability in cultured feline endothelial cells. However, it was not clear if the endothelial cell layers were confluent and the investigators did not show permeability changes were due to VEGF with blocking antibodies or other inhibitory approaches. In other cases, there may be neutrophil chemotaxis without vascular permeability, resulting in high neutrophil counts but a normal protein concentrations. Both are variants of exudative effusions.
The cytologic characteristics of an exudative effusion in small animals are:
- Nucleated cell count: > 5,000/uL. One study, which included septic and non-septic peritonitis, suggested a cut-off of 3,000/uL had the highest diagnostic sensitivity and specificity, compared to other cut-offs (Alonso et al 2021b), as long as the cells in question are inflammatory and not neoplastic in nature (i.e. do not use counts without factoring in smear results).
- Red blood cell (RBC) count: Variable, depending on concurrent hemorrhage or blood contamination.
- Total protein (by refractometer): Usually > 2.5 g/dL, but can be <2.5 g/dL
- Cytologic features: This is variable, depending on the inciting cause. Neutrophils may dominate (>80-85%; in one study of 77 dogs, the median and range of neutrophil percentages was 90%, 56-98% in exudates identified on the basis of nucleated cell counts >5000/μL and total protein ≥2.0 g/dL [Alonso et al 2021a]), indicating suppurative inflammation, or the inflammation may be mixed (neutrophils and macrophages), e.g. feline infectious peritonitis. Neutrophils may be degenerate, particularly with bacterial causes. Protein crescents may be seen when the protein is quite high. A search for the cause of inflammation should be conducted, e.g. infectious agents, bile, underlying neoplasia. At Cornell University, we routinely perform gram stains on exudative effusions. As for other effusions, low numbers of mast cells, eosinophils and reactive lymphocytes or plasma cells are seen. Note, that there are some eosinophilic-rich effusions. We always consider an underlying mast cell tumor or lymphoma, as well as migrating parasites and certain infectious organisms (e.g. fungi), but eosinophils are not specific as to the cause of the effusion.
Hemorrhagic effusion
At Cornell University, we reserve the term “hemorrhagic effusion” for cases in which hemorrhage is considered to be the primary cause for the effusion (e.g. hemostatic disorder, traumatic or benign hemoabdomen, or ruptured hemangiosarcoma) versus hemorrhage accompanying or complicating an effusion from other causes. With hemorrhagic effusions, the PCV of the effusion fluid is usually measurable (>1%) with RBC counts in the millions/ul. Whenever there are RBCs in an effusion, we try to distinguish whether the RBCs are present from blood contamination during collection or from true hemorrhage into the cavity. There are several ways to try to make this distinction:
- During collection of the sample: If the fluid changes from red to clear or clear to red during collection, it suggests the blood is present from contamination as the needle passes through a vessel. If the entire sample is uniformly red or brown as it is collected, it suggests real in vivo hemorrhage. This information is only apparent to the clinical pathologist if provided on the request form.
- Collection into a non–anticoagulant (red top) tube: If the fluid is bloody, collection of fluid into a non-anticoagulant tube can be helpful. The fluid will clot if there is blood contamination or inadvertent aspiration of the spleen. The fluid should not clot with a hemorrhagic effusion (see below for postulated mechanisms).
- During cytology smear evaluation: Here we look for the following:
- Platelets: During hemorrhage into a body cavity, platelets are activated and consumed, so few platelets may be present. If platelets are present on the smear, it means that there is blood contamination or acute or ongoing hemorrhage – so the lack of platelets is more helpful than their presence.
- Evidence of RBC phagocytosis and destruction in macrophages – erythrophages, hemosiderophages, hematoidin crystals: Fairly quickly after hemorrhage begins (probably within a few hours in some cases), macrophages will start to phagocytize free RBCs. However, this can also occur as an in vitro artifact if the sample sits in the tube for a few hours before making the slides (e.g. the sample is sent to the lab overnight). We have observed that in vitro erythrophagia can occur within 2 hours of sample collection (and may occur quicker if macrophages are already “activated” in vivo). So erythrophagia is more pertinent in a freshly made smear of the fluid and less conclusive in a smear made from stored fluid. After erythrophagia, the macrophages take time (probably at least a day or two) to break the RBCs down into hemosiderin or hematoidin (a form of bilirubin, created in low oxygen environments). Thus, if hemosiderin or hematoidin is present on the smear, it indicates hemorrhage of some duration (and confirms in vivo hemorrhage or RBC extravasation even in a stored sample). If the hemorrhage is ongoing, erythrophages and possibly platelets will be seen.
The lack of clotting when a hemorrhagic effusion is collected into a red top tube is a well-recognized phenomenon. A study in 1983 in which radiolabeled fibrinogen was infused with 10 ml/kg of blood via a femoral vein to peritoneal shunt into dogs, showed that the fibrinogen was rapidly degraded to fibrin(ogen) degradation products within 1 hour of infusion, with no crosslinked products being formed. The cleavage of fibrinogen was completely inhibited by heparin, suggesting that thrombin is activated and the blood rapidly clots once infused into the cavity, but then is rapidly lysed. Administration of the fibrinolytic inhibitor, ε-aminocaproic acid, caused clotting of the blood, suggesting that fibrinolysis is the main reason why a hemorrhagic effusion does not clot (Moore et al 1983). Canine mesothelial cells do demonstrate fibrinolytic activity in vitro (Louagie et al 1986). Cultured human mesothelial cells express tissue plasminogen activator (will stimulate fibrinolysis) and plasminogen activator inhibitor-1 (will inhibit fibrinolysis), so the balance of these two mediators will dictate if the mesothelium is profibrinolytic or antifibrinolytic (promoting adhesions) (van Hinsbergh et al 1990). There may also be regional differences in fibrinolytic activity of the mesothelium (Merto et al 1980).
When there is hemorrhage into the peritoneum, the red blood cells and plasma is reabsorbed, largely intact, by abdominal lymphatics, helping to restore blood volume and circulating red blood cell numbers. In one study, where dogs were given autologous blood into the peritoneum (between 20 and 500 ml), most of the red blood cells were resorbed within 24 hours (up to 80%) with <10% remaining at 96 hours. Based on radiolabeling, the infused red blood cells were identified in the systemic circulation and had a mean half life of 21 days, which is shorter than normal red blood cells (this may be a consequence of radiolabeling) (Allcock 1962).
Causes of a hemorrhagic effusion include:
- Trauma: Ruptured vessel (e.g. uterine artery in mares after foaling [Conwell et al 2010]), spleen, kidney or liver (Mongil et al 1995, Dechant et al 2006, Fleming et al 2018)
- Ruptured splenic hematoma: Benign hemorrhagic peritoneal effusion (most commonly seen in dogs) (Mongil et al 1995, Culp et al 2010, Fleming et al 2018)
- Ruptured liver from amyloidosis in cats (Godfrey and Day 1989, Beatty et al 2002)
- Cancer-associated hemorrhage: Rupture of a splenic hemangiosarcoma (Schick et al 2019, Culp et al 2010), vascular invasion and erosion of other aggressive neoplasms (Luethy et al 2016, Fleming et al 2018)
- Coagulopathy: Usually severe and involving secondary hemostasis, e.g. anticoagulant rodenticide toxicosis (Duvall et al 1989, Carvallo et al 2015). Animals with inherited defects in the intrinsic and common pathways may develop hemoperitoneum with elective or emergency surgeries (Waddell et al 2013, Heuss and Weatherton 2016). Animals with severe thrombocytopenia or primary hemostatic defects, e.g. von Willebrand disease, are less likely to spontaneously bleed into the abdomen, but may show hemoperitoneum with surgery or after trauma.
- Idiopathic hemoperitoneum in horses (Dechant et al 2006, Conwell et al 2010)
- Anaphylaxis in dogs (may be due to concurrent coagulopathy or vascular dysfunction) (Hnatusko et al 2021, 2021 diagnostic challenge #4).
Biliary rupture/leakage
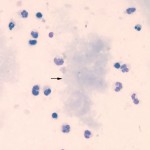
Bile leakage into the abdominal cavity can occur with trauma, ruptured mucoceles (dogs), obstructive lesions (e.g. cholelithiasis) and inflammation or neoplasia in the biliary system or liver. Material resembling bile can also be seen in effusions secondary to rupture or leakage of the duodenum. The bile fluid itself is responsible for only a small amount of the resulting effusion, with the effusion being mostly caused by the ensuing inflammatory response that bile elicits (it is an emulsifying agent, after all). Bile may be recognized as yellow-brown material either free or within macrophages (bile within macrophages may be difficult to distinguish from hemosiderin) or as “white” bile, which is characterized by aggregates or streaks of mucus (small amounts of which are difficult to distinguish from fibrin) (Owens et al 2003). In most cases, biliary rupture can be confirmed by measurement of bilirubin in the fluid, which would be higher than serum bilirubin (usually 2x or more higher), however we have seen cases of ruptured mucoceles, consisting of white bile, in which the total bilirubin concentration is less than that observed in serum.
Uroperitoneum
Urine leakage into the abdominal cavity can occur with trauma, severe inflammation, neoplasia, or obstruction (urolithiasis) within the urinary tract (bladder or uterers). If the rupture is large, the fluid would be very similar to urine (with fairly low cell counts and total protein) immediately after the rupture. However, similar to biliary rupture, the free urine will then elicit an inflammatory response causing an exudative effusion. Similarly, a small lesion allowing urine to leak into the abdomen will induce a sterile peritonitis (unless there is a concurrent urinary tract infection). A uroperitoneum can be confirmed by comparing creatinine levels in the fluid and serum (usually >2x higher in the fluid than serum). Although urea nitrogen concentrations are also likely to be high initially, urea is a small highly diffusable molecule so will equilibrate rapidly with serum urea, making it less useful than creatinine for confirming a uroperitoneum.
GI rupture/leakage
This can occur with trauma, obstructive, inflammatory or strangulating lesions, neoplasia, or a foreign body. Similarly to biliary rupture and uroperitoneum, the majority of the effusion is caused by the resulting inflammation and exudation, with bacterial flora helping to trigger the inflammatory response. The presence of a mixed population of bacteria in neutrophils (including species normally found in the intestinal tract, such as Enterococcus or Peptostreptococcus) or food material on the cytologic smear can help confirm the rupture. However, accidental sampling of intestinal contents during fluid collection (enterocentesis) can be confused with a GI rupture. With enterocentesis, we do not expect to see an inflammatory response or intracellular bacteria and no peritoneal fluid cavity cells are seen. This does mimic samples collected immediately after a GI rupture (particularly if a large amount of GI fluid is released), the cytologic results may be indistinguishable from enterocentesis, since there has not been sufficient time for an inflammatory response. In these cases, GI rupture may be clinically suspected due to rapid deterioration of the patient, warranting either immediate surgical intervention or resampling after a few hours depending on the clinical severity of the patient’s illness. This illustrates that cytologic results should never be interpreted in isolation, but always in context of the patient.
Neoplastic effusions
Some neoplastic processes can lead to increased volumes of body cavity fluid, due to a variety of mechanisms (more than one mechanism may be operative with one tumor):
- Compression of lymphatics by the tumor: Chylous or lymphocyte-rich effusion, e.g. thymoma, thymic lymphoma
- Venous compression causing increased hydrostatic pressure: Transudative effusion
- Blood vessel erosion/invasion or rupture of a vascular tumor: Hemorrhagic effusion, e.g. hemangiosarcoma
- Cytokine or vasoactive mediator production by tumor cells increasing vascular permeability: Exudative effusion
- Inflammation: Exudative effusion
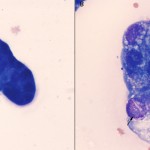
Not all types of tumor will exfoliate into effusions, thus tumor cells may or may not be identifiable in the fluid. Therefore, underlying neoplasia can rarely ever be ruled out as a cause for an effusion. Lymphoma and carcinomas are more likely to release diagnostic cells into cavity fluid than sarcomas (e.g. hemangiosarcoma). Lymphoma typically exfoliates large numbers of cells into the fluid. Neoplastic cells can be readily identified if they are large, however if the lymphoma is comprised of small cells or only exfoliates low numbers of cells, the diagnosis may be more difficult and could readily be missed without more advanced diagnostic procedures, e.g. clonality testing. Carcinomas can be identified in fluids when there are clusters of adherent cells (some cells may be individualized), demonstrating cytologic criteria of malignancy, e.g. anisocytosis, anisokaryosis, multiple bizarre nucleoli, mitotic figures, etc. However, it can be very difficult to distinguish carcinoma cells from non-neoplastic reactive mesothelial cells (since all of these cells are often in clusters) and impossible to distinguish from mesothelioma, but luckily, the latter are rare.
Related links
- Table summary and diagnostic algorithm for effusions.
