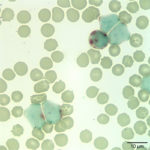
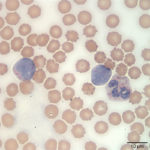
Interpretation
Peripheral blood: Acute leukemia or leukemic phase of precursor lymphoma (Question 1)
Pleural fluid: Neoplastic effusion with concurrent hemorrhage or red blood cell diapedesis
Explanation
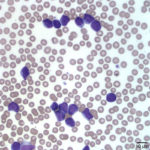
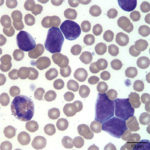
The white blood cells counted as “other” consisted of blasts (Figures 1 and 2). Although the majority of these cells were small to intermediate in size (with occasional large forms present), nuclear features of fine chromatin and indistinct nucleoli identified the cells as blasts. The large number of blasts in blood was consistent with an acute leukemia. However, given the lack of significant cytopenias, the presence of mediastinal mass, and pleural effusion, a leukemic phase of precursor lymphoma was not ruled out. The anemia was mild and non-regenerative and interpreted to be secondary to suppressed erythropoiesis in response to inflammation or leukemia (cytokine-mediated or altered marrow environment and possible myelophthisis). The left shift and toxic change in neutrophils indicated inflammation and/or cytokine-induced granulopoiesis secondary to leukemia. The mild to moderate thrombocytopenia was considered to be either breed-related (which would explain the increased MPV and macroplatelets) or decreased production associated to leukemia. Consumption, abnormal production and peripheral destruction were also considered possible.
The majority of the cells observed on cytologic examination of the pleural fluid were similar to those present in blood indicating a neoplastic effusion. Erythrophagia (not shown) and low numbers of platelets were observed, indicating recent hemorrhage or red blood cell diapedesis, or tap-related blood contamination.
Additional Diagnostic Testing
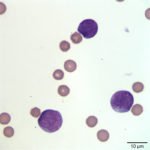
To determine the type of hematopoietic neoplasm, flow cytometry analysis and cytochemical staining of the peripheral blood and pleural fluid were performed (Question 2). Results were similar across specimens in each of the tests. Flow cytometric analysis revealed a population of abnormal cells in the R1 and R2 gates (Figure 4), consisting of intermediate- and large-sized cells, respectively. The cells were labeled against several antigens and a summary of the results are shown in the table below.
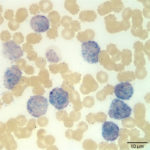
The cells were negative for major histocompatibility antigen II (MHCII, monocyte and lymphocyte), CD3 ( T cell), and B cell markers (CD21 and CD22). The immunophenotypic findings were most compatible with an acute myeloid leukemia (AML). The expression of stem cell antigens, CD34 and CD90 with concurrent lack of MHCII, supported a diagnosis of acute leukemia versus lymphoma.1 The concurrent expression of myeloid antigens, CD11b2,3 (a marker of monocytes and neutrophils) and CD80 (a marker of monocytes), supported myeloid differentiation (i.e. AML). Aberrant generally weak CD5 expression has been reported in some cases of AML.4 Cytochemical staining results were additional support of an AML. On cytochemical staining, 100% of the blasts were moderately positive for alkaline phosphatase (ALP, Figure 5, a marker of canine monoblasts), 74% showed diffuse to focal or chunky cytoplasmic staining (consistent with a monocyte staining pattern) for alpha naphthyl butyrate esterase (ANBE, Figure 6), and 4% were positive for chloroacetate esterase (CAE, Figure 7, a marker of neutrophils in the dog).
|
|
|
|
Case follow-up
Bone marrow collection for cytologic examination (Question 2) was not done due to an increased risk of death under general anesthesia. A therapeutic thoracocentesis was performed and oxygen supplementation was discontinued due to improved blood oxygen saturation. Chemotherapeutic options and prognosis were discussed with the owners, who decided for palliative care with prednisone, Cerenia, and Clavamox. The dog was discharged without any further diagnostic work-up, with instructions to closely monitor for signs of respiratory distress and periodic examination by the primary veterinarian. The dog remained stable and showed marked clinical improvement for up to three months, by which time, blood work revealed significant worsening cytopenias and pancytopenia. Palliative treatment was continued with an increased dosage of prednisone. One month after the final visit to Cornell University, the dog was humanely euthanized by the primary veterinarian due to worsening of clinical signs and disease progression.
Discussion
Acute myeloid leukemia (AML) arises from hematopoietic stem cells in the marrow and gradually replaces and effaces the marrow. On presentation, dogs typically have advanced disease with substantial marrow involvement, which manifests as moderate to severe bicytopenia or pancytopenia. This dog presented with mild anemia and thrombocytopenia, both of which could potentially be attributed to other causes (inflammatory disease and breed, respectively). However, the marked leukemia was concerning for an acute leukemia, which unfortunately was not confirmed by bone marrow aspiration. There are two possible explanations for the mild cytopenias: 1) The bone marrow was not completely effaced or 2) The blasts came from an extramedullary site (myeloid sarcoma), despite being of myeloid origin. Acute myeloid leukemia arising from extramedullary sites has been reported in humans with myeloid sarcoma; a rare tumor that either precedes (e.g. isolated myeloid sarcoma without leukemia) or follows the diagnosis of AML.5 There are only a few case reports of myeloid sarcomas in animals and the association with progression to AML is unclear.6 These tumors are typically found in skeletal muscle in cattle and the lung, gastrointestinal tract, lymph node, liver or skin in dogs or cats.6 However, it can arise in any site. Unfortunately, we did not get a direct aspirate of the mediastinal mass for cytologic examination to confirm the possibility of a mediastinal myeloid sarcoma, but we have seen several cases of AML with mediastinal masses, some of which were confirmed to be AML on cytologic or histologic examination and phenotyping. The neoplastic pleural effusion suggested the mediastinal mass may be the primary site of origin, however. Although not a typically reported location for myeloid sarcoma, there are reports of myeloid sarcoma arising from the mediastinum in humans and animals.6,7 Alternatively, the mediastinal mass and subsequent pleural effusion could have been secondary to extramedullary spread from a typical AML arising in the marrow.
Distinction between types of acute leukemia is important, given that acute lymphoblastic leukemia (ALL) may respond better to aggressive chemotherapy than AML. In acute leukemia however, the tumor cells may or may not show features of differentiation, thus morphologic features alone should not be used to differentiate AML from ALL. Immunophenotyping via flow cytometry and cytochemical staining can help us differentiate between the types of leukemia and potentially achieve a more definitive diagnosis. Results from these tests should be interpreted in combination with clinical data, hematologic and bone marrow findings, and not in isolation.
Three months after initial presentation, there were worsening cytopenias, which we attributed to disease progression and likely marrow effacement by neoplastic cells. Unfortunately, a bone marrow aspirate was not done to confirm this suspicion, however humans with isolated myeloid sarcomas typically progress to blood and marrow involvement over a 10- to 12-months period.5 The prognosis in humans with myeloid sarcoma is poor if left untreated, with most patients succumbing to disease within a short period, with the duration of survival based on the site of involvement.5 Data about prognosis and disease progression of myeloid sarcoma in animals are lacking.
In conclusion, this case represents an unusual presentation of AML, with a relative lack of substantial cytopenias and a pleural effusion and mediastinal mass that mimicked lymphoma. The large number of blasts in circulation, in conjunction with the immunophenotyping and cytochemical results, was most compatible with an acute myeloid leukemia. The initial lack of significant cytopenias, the presence of a mediastinal mass and blasts in the pleural effusion suggested the possibility of a myeloid sarcoma in the mediastinum as the primary site of origin, which ultimately led to worsening cytopenias and euthanasia of this patient. This case also emphasizes the importance of using adjunctive diagnostic tools such as immunophenotyping and cytochemical staining to differentiate between types of acute leukemia.
References
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89(7):2804–8.
- Danilenko DM, Moore PF, Rossitto P V. Canine leukocyte cell adhesion molecules (LeuCAMs): Characterization of the CD11/CD18 family. Tissue Antigens. 1992;40(1):13–21.
- Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69(2–4):145–64.
- Stokol T, Schaefer DM, Shuman M, Belcher N, Dong L. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Pathol. 2015;44(1):79–93.
- Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk [Internet]. 2017;1–5. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2152265016306309
- Morosco DT, Cline CR, Owston MA, Kumar S, Dick EJ. Spontaneous mediastinal myeloid sarcoma in a common marmoset ( Callithrix jacchus ) and review of the veterinary literature. J Med Primatol [Internet]. 2017;46(2):42–7. Available from: http://doi.wiley.com/10.1111/jmp.12253
- Kant Sahu K, Ruchita Tyagi B, Arjun Datt Law B, Alka Khadwal B, Prakash G, Arvind Rajwanshi B, et al. Myeloid Sarcoma: An Unusual Case of Mediastinal Mass and Malignant Pleural Effusion with Review of Literature. Indian J Hematol Blood Transfus [Internet]. 2015;31(4):466–71. Available from: http://dx.doi.org/10.1007/s12288-015-0536-z
Authored by: D. Hernandez and T. Stokol