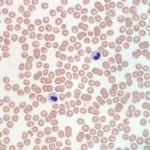
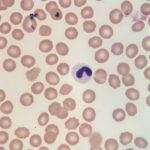
Interpretation
Lymphoma, megakaryocytic hypoplasia and Pelger-Huët anomaly.
Explanation
The bone marrow sample was hypercellular, with minimal fat present. This hypercellularity was due to a population of neoplastic cells (Question 1). The tumor cells were found in diffuse sheets but also in patches in the marrow. Overall, they comprised greater than 25% of cells in the marrow aspirate. The tumor cells were approximately 1.5 times the size of a neutrophil (large), had a round to indented nucleus with fine chromatin and 1-2 indistinct nucleoli (in some cells) and contained a small amount of medium blue cytoplasm. The neoplastic cells in bone marrow were similar to those seen in peripheral blood and lymph node aspirates. They were suspected to be of lymphoid origin. Only low numbers of megakaryocytes were seen (not pictured), which indicates an inadequate marrow response to the marked thrombocytopenia in peripheral blood. This observation was interpreted as megakaryocytic hypoplasia. Erythroid and myeloid precursors were present, but in low numbers, suggesting a concurrent erythroid and myeloid hypoplasia. These findings, however, should be interpreted with respect to peripheral blood results. The dog was not anemic, however an erythroid hypoplasia may take time to manifest due to the long red blood cell lifespan. Neutrophil numbers were within normal limits, which argues against a myeloid hypoplasia suggesting that marrow production of these cells was adequate. The marrow iron content appeared subjectively normal to mildly decreased (this was performed on a Prussian blue-stained smear). The lack of marrow iron could be secondary to subclinical blood loss from the severe thrombocytopenia or consumption by the rapidly dividing tumor cells (suspected explanation).
Based on the peripheral lymphadenopathy and presence of tumor cells in the lymph node aspirates and solitary cytopenia in blood, a diagnosis of lymphoma was initially made in the bone marrow aspirate. However, the heavy infiltration in the bone marrow raises the possibility that this case was an acute lymphoid leukemia (ALL) that was secondarily infiltrating the lymph nodes (which can occur with any type of primary leukemia of lymphoid or myeloid origin, whether comprised of immature/precursor [acute] or mature [chronic] cells).
Lymphoma and lymphoid leukemia (mature or precursor variants) are considered to represent each end of a spectrum of lymphoid neoplasia. At one end of the spectrum, there is lymphoma, which is a tumor that arises in extramedullary sites, such as peripheral lymph nodes. On the other end of the spectrum, there is lymphoid leukemia, that arises in the marrow in the case of ALL. Between these two ends of the spectrum, there is a range of presentations, where you may have a primary ALL infiltrating lymph nodes or other organs (liver, spleen particularly) or lymphoma with circulating tumor cells (aka “lymphoma cell leukemia”) or that infiltrates the marrow. This dog falls into the “grey zone” or between the two spectrums. How do we distinguish if this is lymphoma or an ALL? There are some tools that we can use to help us with this decision (Question 3).
- Extent of bone marrow involvement: World Health Organization (WHO) criteria for precursor lymphoid neoplasms1 distinguish ALL from lymphoma on the basis of percentage of marrow tumor cells. If there are >25% tumor cells in marrow, the diagnosis of precursor lymphoid leukemia or ALL is made. Since the bone marrow aspirate in this dog had >25% of tumor cells, the dog would be diagnosed with ALL by WHO criteria. However, the patchy infiltrate of the tumor cells in marrow (weakly) suggested an infiltrative versus primary bone marrow tumor.
- Expression of stem cell markers with flow cytometry (Question 3): Expression of the stem cell marker, CD34, would favor a diagnosis of ALL, however this cannot be relied upon for T-ALL, which often do not express stem cell markers.2,3 Furthermore, we and others4 have found that some B cell lymphomas can weakly express CD34. One would also expect T-ALL to be double negative for CD4 and CD8 (since these markers are acquired in the thymus), but in reality some T-ALL can be double or singly positive for CD4 and CD8.1 Flow cytometric analysis of a peripheral lymph node aspirate in this case revealed that the neoplastic cells were positive for CD45 (common leukocyte antigen), CD3 (T cell marker), MHCII and CD4 (helper/regulatory T cell marker). They were negative for CD34 and CD5 (T cell marker, aberrant expression).
- Severity of cytopenias: ALL is more likely to induce multiple and severe cytopenias (due to extensive marrow involvement or myelophthisis). This dog only had solitary thrombocytopenia. In our experience, an unexplained thrombocytopenia (and sometimes neutropenia) can be found in dogs and horses with lymphoma. However, not all animals with acute leukemia (whether myeloid or lymphoid) have a concurrent bi- or pancytopenia. Thus, the lack of anemia and neutropenia may (weakly) argue for a primary lymphoma in this case.
- Other findings: Hypercalcemia is more common with T cell lymphoma than T-ALL.
- Main site of involvement: Ultimately, the final diagnosis of lymphoma versus ALL will rely on determination of the main site of involvement – extramedullary site for lymphoma or bone marrow for ALL. In this case, this is difficult because some but not all of the peripheral lymph nodes were enlarged and the bone marrow was substantially infiltrated, which would favor a diagnosis of ALL.
If we take all the results of this dog together, more things “fit” with lymphoma than ALL – hypercalcemia, solitary cytopenia, peripheral and internal lymphadenopathy, and patchy, but extensive, infiltrates in marrow. Even though there was relative myeloid and erythroid hypoplasia in the bone marrow, production of erythrocytes and neutrophils appeared unimpaired based on counts in peripheral blood. Indeed, the flow cytometric results support a diagnosis of CD4+ peripheral T cell lymphoma, as reported by Avery et al (2014).5 These lymphomas can behave in an aggressive fashion and decreased expression of CD5 and large cell size (both seen in this case) are considered negative prognostic indicators. Hypercalcemia and mediastinal masses have also been identified in 50% and 43% of the cases, respectively.5
The thrombocytopenia in this case was attributed to the underlying lymphoid neoplasia (Question 2). Neoplasia may result in thrombocytopenia via several mechanisms, including: consumption (e.g. disseminated intravascular coagulation), decreased marrow production secondary to myelophthisis (possibly occurring in some areas of the marrow) and secondary immune-mediated destruction, which may be directed toward mature platelets or megakaryocytes.6,7 Other contributing factors could include sequestration of platelets in the spleen (if affected by diffuse infiltrative disease) and decreased platelet life span, which could also be due to immune-mediated mechanisms.6,7 The megakaryocytic hypoplasia suggests a decrease in platelet production was contributing to the peripheral thrombocytopenia. The cause of the hypoplasia is unknown but it could be secondary to direct suppresssive effects or possible immune-mediated destruction induced by the tumor. A contribution from concurrent platelet activation and consumption secondary to non-overt disseminated intravascular coagulation cannot be completely ruled out (coagulation testing was not done in this dog).
Also noted on evaluation of the bone marrow sample was atypical nuclear morphology of the neutrophils and eosinophils. Despite the mature, highly clumped chromatin, the vast majority of the granulocytic cells present in the marrow retained a band, linear or round shaped nucleus and were generally hyposegmented (Figures 2 and 3). These morphologic features were also evident in granulocytes in peripheral blood (Figures 4 and 5). No toxic change to help support accelerated granulopoiesis due to an inflammatory response was evident. The combination of these findings (mature chromatin but immature nuclear shapes with no toxic change) in this breed of dog, is indicative of Pelger-Huët anomaly (Question 4). Pelger-Huët anomaly is not considered to be clinically significant, but the atypical neutrophil morphology must be differentiated from left-shifted neutrophils to avoid the incorrect diagnosis of an inflammatory leukogram (Question 4).
|
|
|
Discussion (of Pelger-Huët anomaly)
Pelger-Huët anomaly is an inherited disorder, which results in the hyposegmentation of neutrophils and eosinophils. Occasionally, mild changes may be noted in the nuclear shape of monocytes and megakaryocytes, as well.8,9 In human beings and mice, this anomaly is due to a mutation in the gene that encodes for the lamin B receptor located within the inner nuclear membrane.10,11 This receptor is involved in the transport of lamins (structural intermediate filaments) to the nuclear membrane, which presumably contributes to the development of nuclear segmentation. Defects in the lamin B receptor therefore result in nuclear hyposegmentation.10,11 The nuclear morphology of affected granulocytes is variable and nuclei may appear as band-like, round, oval or sometimes bilobed or peanut-shaped.
This condition has been reported in dogs, cats, rabbits, humans and mice.8,12 In most species, Pelger- Huët anomaly has an autosomal dominant inheritance pattern. Most heterozygotes exhibit the above morphological changes, but are otherwise normal, healthy individuals. The affected neutrophils are functionally normal. The homozygous form is rare and has been reported in humans, rabbits and cats. This form is typically lethal in veterinary species, resulting in late term death of affected fetuses and severe chondrodysplasia.13 Pelger-Huët anomaly has been reported in many dog breeds, including, but not limited to, Australian shepherds, foxhounds, Basenjis, Samoyeds and mixed breed dogs.14,15 Australian shepherd dogs are somewhat unique, in that the mode of inheritance appears to also be autosomal dominant, but with incomplete penetrance.14 There also appears to be a high incidence of Pelger-Huët anomaly in this breed, as it was identified in 9.8% of Australian shepherds in a region of California.14
It is important to identify Pelger-Huët anomaly in affected individuals to avoid the misdiagnosis of a left shift in peripheral blood, which could lead to an erroneous diagnosis of a severe underlying infection and unnecessary diagnostic tests or treatment. Truly left-shifted neutrophil nuclei have a horseshoe to bean shape with a finer chromatin pattern than is exhibited by Pelger-Huët neutrophils. Left-shifted neutrophils also typically exhibit toxic change. Uncommonly, neutrophils may take on the appearance of Pelger- Huët neutrophils due to an underlying myelodysplastic syndrome, exposure to certain drugs (e.g. sulfadiazine-trimethoprim) and some infections.8,15 This acquired form, or pseudo-Pelger-Huët anomaly, can be ruled out based on the overall clinical findings and history. Additionally, pseudo-Pelger-Huët neutrophils will retain a slightly finer chromatin pattern than true Pelger-Huët neutrophils. We have rarely identified this reported pseudo-Pelger-Huët variant in animals. Pelger-Huët anomaly can typically be diagnosed via blood film examination by trained personnel, in conjunction with the overall case information. Bone marrow examination is not necessary to make the diagnosis, but provided an aesthetically pleasing sample in this case.
Additional information
The dog in this case was treated with intravenous fluids and symptomatically for diarrhea and nausea, which improved the dog’s overall energy level and appetite. The dog was released to the care of its owners. Initially, only prednisolone was prescribed to target the underlying lymphoma while the owners contemplated additional chemotherapy. Two weeks after the initial discharge, the dog returned to Cornell University Hospital for Animals to begin multi-drug chemotherapy, at which time the platelet count had increased to 75,000/μL and no circulating neoplastic cells were noted in circulation. The dog was clinically much improved.
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumors of haematopoietic and lymphoid tissue, 2008, 4 ed. International Agency for Research on Cancer (IARC), Lyon, France
- Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145-164.
- Stokol T, Schaefer D, Bell T, Shuman M, Dong L (2015) Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. 2015;44:77-93.
- Wilkerson MJ, Dolce K, Koopman T, et al. Lineage differentiation of canine lymphoma/leukemia and aberrant expression of CD molecules. Vet Immunol Immunopathol. 2005;106:179-196.
- Avery PR, Burton J, Bromberek JL, et al. Flow cytometric characterization and clinical outcome of CD4+ T-cell lymphoma in dogs: 67 cases. J Vet Intern Med. 2014;28:538-546.
- Schwartz KA, Slichter SJ and Harker LA. Immune-mediated platelet destruction and thrombocytopenia in patients with solid tumors. Br J Haematol. 1982;51:17-24.
- Botsch V, Kuchenhoff H, Harmann K, et al. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec. 2009;164:647-51.
- Weiss DJ. Neutrophil function disorders. In: Weiss DJ and Wardrop KJ, ed. Schalm’s Veterinary Hematology. Ames, IA; Blackwell, 2010;275-80.
- Latimer KS, Duncan JR and Kircher IM. Nuclear segmentation, ultrastructure and cytochemistry of blood cells from dogs with Pelger-Huet anomaly. J Comp Path. 1987;97:61-72.
- Best S, Salvati F, Kallo J, et al. Lamin B-receptor mutations in Pelger-Huet anomaly. Br J Haematol. 2003;123:542-4.
- Schultz LD, Lyons BL, Burzenski LM, et al. Mutations at the mouse ichthyosis locus are within the lamin B receptor gene: a single gene model for human Pelger-Huet anomaly. Hum Mol Genet. 2003;12:61-9.
- Ye Q, Callebaut I, Pezhman A, et al. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J Biol Chem. 1997;272:14983-9.
- Latimer KS, Rowland GN and Mahaffey MB. Homozygous Pelger-Huet anomaly and chondrodysplasia in a stillborn kitten. Vet Pathol. 1988;25:325-8
- Latimer KS, Campagnoli RP and Danilenko DM. Pelger-Huet anomaly in Australian shepherds: 87 cases (1991-1997). Comp Haematol Inter. 2000;10:9-13.
- Vale AM, Tomaz, KLR, Sousa RS, et. al. Pelger-Huet anomaly in two related mixed-breed dogs. J Vet Diag Invest. 2011;23:863-5.
Authored by: Dr. L. Brandt and T Stokol