Interpretation
Urinary tract obstruction with post-renal azotemia, a mixed acid-base disturbance (primary metabolic alkalosis and primary titration metabolic acidosis) and an inflammatory leukogram.
Explanation
Complete Blood Count:
There was a mild leukocytosis characterized by a mild neutrophilia with a mild left shift and monocytosis. Toxic change was not evident. There was a concurrent lymphopenia and eosinopenia. The left shift, albeit mild, supports an inflammatory leukogram, with a concurrent “stress” response (increased endogenous corticosteroids)1 (Question 1).
Electrolyte abnormalities:
There is a mild hyponatremia with a concurrent moderate hypochloremia. The hyponatremia indicates loss of hypotonic fluids (vomiting of gastrointestinal contents and renal losses, as well as sequestration in a distended bladder). Hypovolemia associated with fluid losses triggers ADH release, activates the renin-angiotensin-aldosterone system and increases thirst. ADH will conserve water in the distal nephron, whereas angiotensin II will stimulate proximal sodium (and water) resorption in the proximal tubules of the kidney. Aldosterone will promote sodium resorption in exchange for potassium excretion in the distal nephron (collecting ducts and tubules). ADH is working to some extent as the urine is concentrated to some degree (although the degree of concentration is inappropriate for the degree of azotemia, indicating defective concentrating ability). The body’s response to hypovolemia (thirst, increased ADH and water retention, increased sodium and water retention) will serve to dilute out the sodium in this case. Note, that even though aldosterone is likely high in this cat, the decreased excretion of potassium due to the urinary tract obstruction is offsetting any visible decrease in potassium by aldosterone-induced excretion (the potassium is not going anywhere).
When interpreting changes in chloride, we must first look at sodium and account for changes in water balance. By eyeballing (comparing results to the lower end of the reference limits for each electrolyte), the decrease in chloride is disproportional to the change in sodium (greater loss of chloride than sodium). One can also correct the chloride for changes in sodium (or water)2 using the following formula (where sodium[normal] is the midpoint of the reference interval):
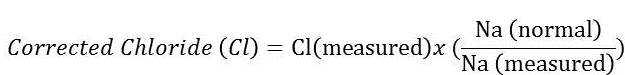 |
The corrected chloride is 110 mEq/L, which is still below the reference interval (113 – 123 mEq/L). Given this result, we can determine that there is a greater loss of chloride than sodium. Tracing back to the cat’s history, the disproportional loss of chloride in relation to sodium can be attributed to vomiting of stomach contents (gastrointestinal losses) with subsequent loss of hydrogen chloride (HCl). Because the chloride is disproportionately low to sodium, the cat has a hypochloremic metabolic alkalosis (Question 2). A metabolic alkalosis can be primary (due to losses of an acid or gain of a base) or secondary to a primary respiratory acidosis. There is no evidence of a respiratory disorder in this cat and with the history of vomiting, it is far more likely that the metabolic alkalosis is a primary acid-base disturbance.
With a metabolic alkalosis, one would expect the bicarbonate to be high. However, the cat conversely has a mild decrease in bicarbonate with a moderate to marked increase in the anion gap (AG). The anion gap, by definition, is the difference between measured cations (Na+ plus K+) and measured anions (Cl– plus HCO3–). This indicates that there is an increase in unmeasured anions or acids (which dissociate and release their hydrogen, which is buffered by bicarbonate and other body buffers, leaving the negatively charged anion to contribute to the anion gap), such as lactate, ketones or uremic acids. Thus in this cat, the low bicarbonate and increased anion gap indicates a concurrent titration metabolic acidosis, which is always a primary acid-base disturbance (Question 2). Due to the marked azotemia, the unmeasured anions that are increasing the anion gap and buffering (decreasing) the bicarbonate are likely “uremic” acids, including phosphates (which is measured on our chemistry panel and is increased due to decreased glomerular filtration rate), sulfates and hippurates. The low bicarbonate suggests that the acidosis is “winning” over the alkalosis, however this would need to be confirmed from a blood gas analysis (to see what the pH is doing, Question 3), which was not done in this cat. So this cat has a mixed acid-base disturbance, i.e. two primary acid-base abnormalities, namely a hypochloremic metabolic alkalosis and a high anion gap titration metabolic acidosis, which will have opposing effects on bicarbonate (alkalosis will increase and acidosis will decrease) and pH (alkalosis will increase and acidosis will decrease).
Another electrolyte abnormality in this cat is a marked hyperkalemia. An increased potassium is uncommon if renal function and urine output are normal.3 In this case, the marked increase in potassium is attributed to impairment of the urinary system to excrete potassium appropriately and is a typical finding in dogs and cats with urinary tract obstruction.3 Due to the hyperkalemia, an electrocardiogram (ECG) was done to evaluate for hyperkalemia-induced cardiac abnormalities (see below), but the ECG was normal (Question 3).
Renal values and urinalysis:

The cat has a marked azotemia characterized by marked increase in urea nitrogen and creatinine with a urine specific gravity (USG) of 1.022. The cat was diagnosed with a lower urinary obstruction, so the azotemia is primarily due to a post- renal (unable to eliminate urine) problem. There is concern for a concurrent renal (kidney damage) component, since the USG is inappropriately concentrated given the severe azotemia. However, pressure related injury to the renal tubules and decreased medullary tonicity from hyponatremia may be impairing the ability of the kidney to respond to ADH. A pre-renal azotemia, given the history of vomiting, might be contributing to some degree of the azotemia, but is not the primary mechanism behind the azotemia. The proteinuria is also excessive for the urine specific gravity but could be secondary to serum proteins accompanying the red blood cells with the gross hematuria (red urine, >100 RBCs/HPF in the sediment). The marked hematuria is likely due to pressure damage generated in the urinary system from the obstruction. Trauma from urinary catheterization is a concurrent possible source of the hematuria, but the hematuria would likely be far less severe. The few epithelial cells are likely transitional in origin and exfoliated from the catheterization procedure. Struvite crystals indicate supersaturation of the urine with ammonium, magnesium and phosphate, which normally precipitates in alkaline urine (the urine pH is neutral to alkaline in this cat). The presence of crystals, albeit few in number, should raise the possibility of a urolith causing renal tract obstruction. Abdominal radiographs were performed to identify a radiodense stone (Question 3), but no uroliths were detected in this case.
Calcium and phosphate:
The mild to moderate hyperphosphatemia is likely due to decreased glomerular filtration rate and renal excretion impairment. The concurrent hypocalcemia cannot be attributed to low albumin (albumin was normal) and likely reflects a low ionized calcium, probably due to complexing to phosphate.4,5 An explanation for the low total calcium is not 100% apparent, but possibilities include impaired activity of PTH, decreased vitamin D synthesis due to renal tubule dysfunction or hyperphosphatemia.5,6 Decreases in total and ionized calcium have been reported in cats with naturally occurring urethral obstruction and the authors speculated that the incidence of low ionized calcium may be higher if the cats were not acidotic (acidosis may cause calcium to detach off albumin).5
Glucose:
The mild increase in glucose could be due to “stress” (endogenous corticosteroids enhancing gluconeogenesis) or epinephrine (causing glycogenolysis).
Aspartate aminotransferase (AST) and creatinine kinase (CK):
Given that the ALT was within the reference interval, the AST is likely coming from muscle because of the concomitant mild increase in CK activity. Mild hemolysis could be increasing the AST activity as well,7 but RBC hemolysis (likely a collection-associated artifact) is unlikely to be the sole cause of the increased AST activity. The source of muscle injury is unknown. It could be smooth muscle injury from bladder distention (less likely as this is not a big muscle mass), but also could be due to a “muscle” stick, which is an artifact of collection (blood is contaminated by muscle “juice”).
Iron and % saturation:
The moderate decrease in iron concentration and percentage saturation is supportive of inflammation due to sequestration of iron from inflammatory cytokine-mediated upregulation of hepcidin. The TIBC is within normal limits because of transferrin’s long half-life, suggesting more recent (acute) inflammation versus a longer-standing process.
Discussion
Feline urinary tract obstruction is a relatively common and potentially life-threatening condition encountered in veterinary medicine. It is the end result of a multifactorial process that is commonly referred to as feline lower urinary tract disease (FLUTD), which is associated with changes in the environment, diet and water intake. Feline urethral obstruction occurs more often in male than female cats, because of the longer and narrower urethra in male cats. Reasons for obstruction include urinary mechanical obstruction (calculi, mucous plug), inflammation (idiopathic cystitis), infection, neoplasia and conformational disorders. Other causes such as urethral spasm and edema are thought to play an equal role in obstructing the urethral lumen and impairing urinary flow. The pathogenesis of idiopathic cystitis, which is the suspected cause of this cat’s problem, is unclear, but it appears to be a sterile inflammatory process, since a viral or bacterial cause is not identified in most cases and some cats, such as the cat in this report, do not have evidence of inflammation in the urine. Studies into neurohumoral alterations in cats with idiopathic cystitis has demonstrated that the disease may be related to an imbalance between the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis brought about by stressful situations.8,9,10 The imbalance is thought to cause impaired blood flow and the release of inflammatory mediators, resulting in edema, smooth muscle spasm and pain in the lower urinary tract.8 Pain, because of smooth muscle dysfunction and bladder distention, generates a vicious cycle that further perpetuates inflammation and edema, which could occur independently or in conjunction with a physical obstruction such as a mucus plug or a stone.8
Clinical signs of patients with urinary obstruction include lethargy, vomiting, anorexia, abdominal pain, vocalization, dysuria, pollakiuria, periuria and straining unproductively in the litter box.11 If the patient is able to dribble urine, there is usually gross evidence of hematuria. Examination findings associated with urinary obstruction include bradycardia (from hyperkalemia), hypothermia, hypotension, abdominal pain and a firm turgid urinary bladder on palpation.10 The degree of distention of the urinary bladder will vary between patients. Mechanically obstructed cats will tend to have a large distended bladder, in comparison to idiopathic cystitis patients, that tend to have a smaller urinary bladder. Other abnormalities encountered, based on the electrolyte (particularly potassium) derangements, are cardiac arrhythmias. An ECG should be performed in cats with a urinary obstruction, as done with the cat of this report (Question 3). ECG changes include a wide P wave, peaked T waves and wide/bizarre QRS complex that may progress to conduction disturbances, escape beats and asystole.11 The electrolyte abnormality most commonly seen with blocked cats is hyperkalemia. The increase in potassium in patients with urinary obstruction is attributed to impaired excretion of potassium by the urinary system. Increases in potassium of 8.0 mEq/L or higher are reported to cause marked weakness in feline patients.3 Bradycardia, weakness or cardiac arrhythmias were not detected in this cat by clinical examination or with the ECG. The time required for development of hyperkalemia in cats after urethral obstruction is variable, but it may occur within 48 hours.3 Hyperkalemia typically resolves within 24 hours once the urethral obstruction is relieved.3
The other electrolytes abnormalities that are not normally associated with urinary obstruction is the disproportionate hypochloremia in proportion to the hyponatremia. The latter electrolytes derangement is classic for an upper gastrointestinal problem, such as vomiting, which this cat had before presentation. The loss of stomach contents and hydrogen chloride caused a drop in chloride to a greater extent than sodium and the loss of hydrogen resulted in a primary metabolic alkalosis. A hypochloremic metabolic alkalosis is an acid-base disturbance commonly associated with patients with upper gastrointestinal problems (vomiting, pyloric partial or complete outflow blockage, etc.). This acid-base disturbance is not typical in patients with urinary obstruction, but in the case of the cat of this report, is likely a sequela of the marked azotemia causing gastrointestinal irritation and therefore vomiting.
The marked azotemia in this case is primarily because of a decrease in urine outflow (post-renal) with a concurrent impairment of excretion of “uremic” acids, such as phosphates. The retention of organic and inorganic acids not being filtered by the kidneys caused a primary high anion gap titration acidosis. Other potential unmeasured anions that might be present in this case are lactic acid, since blocked cats are known to have impaired blood flow to the urinary system,8 which can result in ischemia of the smooth muscle in the urinary bladder, and therefore the buildup of lactic acid. Lactic acid was not measured in this particular case. A blood gas analysis was not performed in this case, but would have been useful to assess the entire acid-base picture in the cat.
The cat was admitted to Cornell University Hospital for Animals for removal of the urinary tract blockage and symptomatic care with intravenous fluids and analgesics to correct the primary acid-base disturbances and help resolve the severe azotemia. The cat was unblocked successfully via urinary catheterization (which gave us urine to analyze) and an indwelling catheter was placed for monitoring urine production over the course of hospitalization. A recent study12 suggests that decompressing urinary cystocentesis can be used to alleviate symptoms in blocked cats, however, this protocol cannot be recommended as an alternative to traditional management because no direct comparison between the two methodologies (urethral catheterization versus decompressing cystocentesis) was made.12 Two days later, a re-check renal panel was submitted to monitor the azotemia and electrolyte derangements. The results are show below.
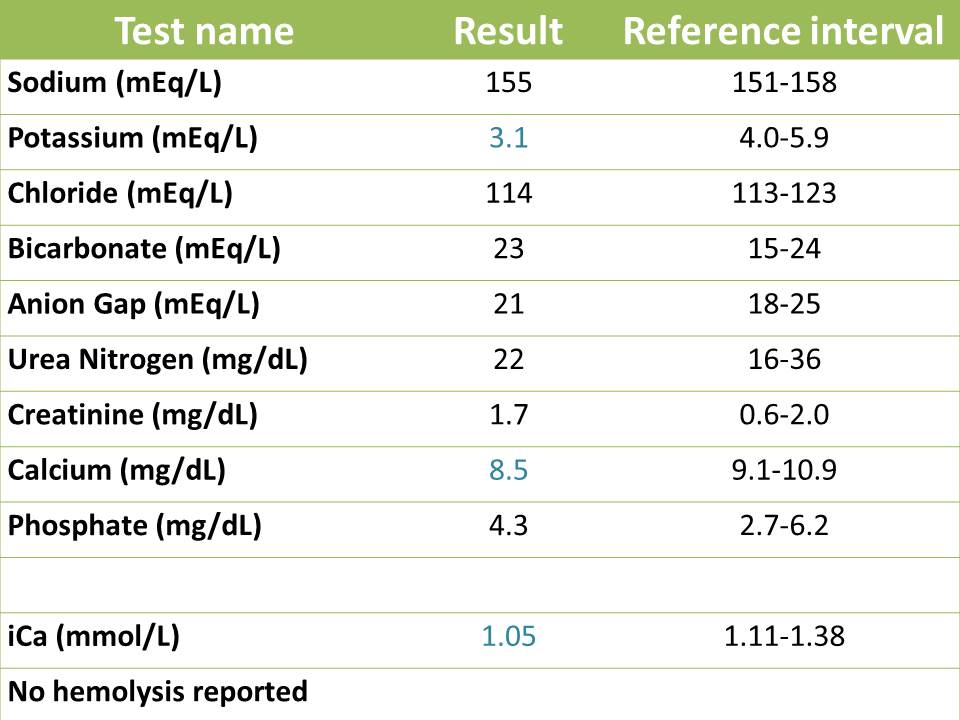 |
On recheck, the marked azotemia had completely resolved, as had the acid-base abnormalities. Typically, azotemia related to urethral obstructions resolves between 24 to 72 hours post de-obstruction3, but this is patient dependent and may take longer. The rapid resolution of the azotemia argues against acute kidney injury secondary to the obstruction. The cat was mildly hypokalemic and hypocalcemic, with low total and ionized calcium. Hypokalemia is a common finding in treated obstructed cats, because of post-obstructive diuresis. Water reabsorption is impaired with obstruction and the diuresis is thought to be secondary to impaired concentration ability of the kidneys, enhanced excretion of retained urea, intravenous fluid supplementation, increased medullary blood flow with IV fluids with subsequent washout of the medullary hypertonic gradient, and an impaired response of collecting ducts to ADH.11 The persistent hypocalcemia suggested continued defects in PTH or vitamin D metabolism. The cat continued to improve and was able to urinate by itself. The animal was discharged to the care of the owner.
In conclusion, urinary obstruction is considered an emergency and life-threatening problem in veterinary medicine. The magnitude and the acuteness of the disease can cause life-threatening changes in electrolytes and acid-base disturbances. It is important that these patients get prompt attention to reduce the side effects that may occur as a sequela of impaired urinary function.
References
- Stockham SL, Scott MA. Leukocytes. In: Fundamentals of Veterinary Clinical Pathology. Blackwell Publishing; 2008. p. 53–106.
- Autran De Morais H, Biondo AW. Disorders of Chloride: Hyperchloremia and Hypochloremia. In: Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice. 4th ed. DiBartola, ed. Elsevier Inc.; 2012. p. 80–91.
- DiBartola SP, Autran De Morais H. Disorders of Potassium: Hypokalemia and Hyperkalemia. In: Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice. 4th ed. DiBartola, ed. Elsevier Inc.; 2012. p. 92–119.
- Schenck PA, Chew DJ, Nagode LA, Rosol TJ. Disorders of Calcium [Internet]. 4th ed. Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice. Elsevier Inc.; 2012. 120-194 p. Available from: http://linkinghub.elsevier.com/retrieve/pii/B9781437706543000135
- Lee J, Drobatz K. Characterization of the clinical characteristics, electrolytes, acid–base, and renal parameters in male cats with urethral obstruction. J Vet Emerg Crit Care [Internet]. 2003;13(4):227–33. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1534-6935.2003.00100.x/full
- Rosol T, Chew DJ, Rosoi TJ, Chew DJ, Nagode LA, Capen CC. Pathophysiology of Calcium Metabolism. 1995;24(November 2016):49–63.
- Stockham SL, Scott MA. Enzymes. In: Fundamentals of Veterinary Clinical Pathology. Blackwell Publishing; 2008. p. 639–74.
- Cooper ES. Controversies in the management of feline urethral obstruction. J Vet Emerg Crit Care. 2015;25(1):130–7.
- Buffington CAT, Teng B, Somogyi GT. Norepinephrine content and adrenoceptor function in the bladder of cats with feline interstitial cystitis. J Urol. 2002;167(4):1876–80.
- Westropp JL, Buffington CAT, Chew D. Feline Lower Urinary Tract Diseases. In: Textbook of Veterinary Internal Medicine. 6th ed. Elsevier Inc.; 2000. p. 1828–50.
- Bartges JW, Finco DR, Polzin DJ, Osborne CA, Barsanti JA, Brown SA. Pathophysiology of Urethral Obstruction. Vet Clin North Am – Small Anim Pract. 1996;26(2):255–64.
- Cooper ES, Owens TJ, Chew DJ, Buffington C a T. A protocol for managing urethral obstruction in male cats without urethral catheterization. J Am Vet Med Assoc. 2010;237(11):1261–6.
Authors: José Daniel Cruz Otero (clinical pathology resident), T. Stokol