Close attention must be paid to all aspects of sample collection, handling and storage, to ensure the most accurate clinical pathologic results. This section provides general guidelines and recommendations for optimally collecting and submitting blood samples for clinical pathologic testing. More detailed information on specific recommendations for individual tests can be found under related sections (hematology, hemostasis, urinalysis, chemistry, cytology) and under each test result. This information includes the effects of controllable preanalytical variables such as how you collect, handle and store the sample before submission to the laboratory. Note that this page refers to blood samples, but some chemistry tests can also be performed in ocular fluids.
Tube types and uses
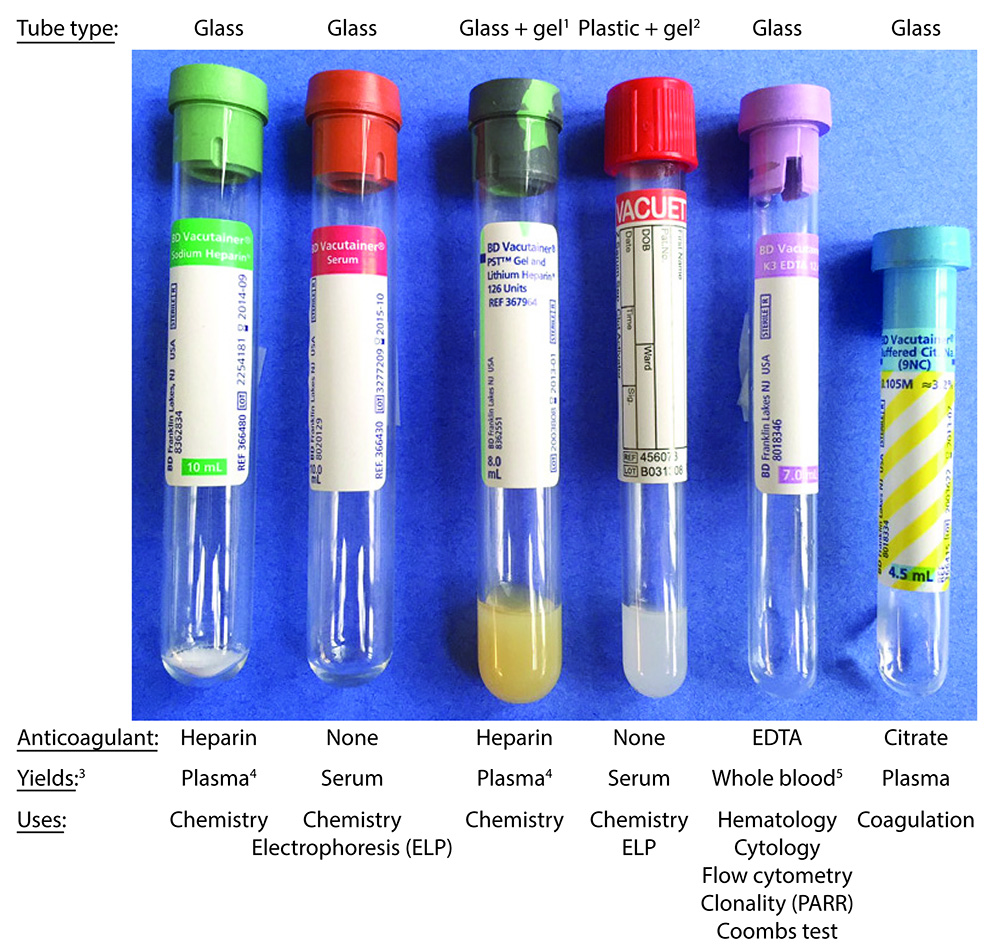
There are many different types of tubes used for clinical pathologic testing, the most common of which are shown in the image below and are: Green top tubes containing lithium heparin (good for stat samples in large animals), red tops with no anticoagulant, purple tops with potassium EDTA as the anticoagulant, and blue tops with citrate as an anticoagulant. Their use for clinical pathologic testing is shown in the image below, including what sample is yielded from the whole blood and used for the testing. All samples for chemistry and coagulation testing should be centrifuged ASAP to yield serum or plasma and not left as whole blood, when shipping samples off to laboratories for testing. EDTA tubes for hematology testing should not be centrifuged at all and should be maintained as whole blood. Some of the chemistry tubes contain silicon gel, which facilitates separation of serum or plasma from cells. These are more commonly used in large versus small animal practice as it can take a while for samples from large animals (horses in particular) to clot and release serum from the clot. Although serum is the general preferred and traditional sample for chemistry testing, we use heparinized plasma (from green top tubes) frequently in large animals, for the ease and rapidity of obtaining plasma from cells as well as the higher yield of plasma, particularly for stat samples. Clinicians also collect blood into 2 heparin microtainers (0.5 ml volume) from each animal for exotics (e.g. birds, small rodents, reptiles) and small animals with expected low blood yields (e.g. puppies, kittens, small dogs or cats). One microtainer rarely provides us with enough volume to run all the tests and perform repeat analyses, as indicated.
The mechanism of action of the anticoagulants are:
- Lithium heparin: Inhibits coagulation factors.
- Potassium EDTA: Chelates calcium strongly
- Sodium citrate: Chelates calcium gently. Calcium is an essential cofactor in coagulation and we have to add it back to perform clotting or coagulation assays, such as the prothrombin time and activated partial thromboplastin time.
 |
|
Collection of blood
- Clean venipuncture is essential to minimize artifactual changes in the results. Blood should flow freely with minimal interruptions during collection. This is particularly important for hemostasis testing.
- Anticoagulant (see above)
- Hematology: EDTA is preferred in most species, although blood will lyse in EDTA in certain species of birds (e.g. cranes, crows, turkeys, hornbills, wood ducks) and reptiles (e.g. tortoises). Always submit a freshly made blood smear (preferably unstained) to optimize examination of red blood cell, white blood cell and platelet morphologic features.
- Hemostasis: Citrate anticoagulant (blue top) is optimal for coagulation assays, whereas EDTA is required for platelet counts. Strict attention must be paid to collection (correct volume, clean venipuncture as detailed more in the related section).
- Urinalysis: Samples should be collected into sterile containers. Plastic non-anticoagulant tubes should be avoided (contains crystalline material which introduces artifact); glass non anticoagulant tubes (red top) are fine.
- Chemistry: Either non-anticoagulant (red top) or heparin anticoagulant (green top can be used). Most laboratories prefer serum and ancillary tests are usually done on serum (e.g. infectious disease testing). We prefer heparinized blood for stat samples as the plasma can be harvested quickly. Results in plasma and serum differ slightly for some analytes (e.g. total protein and potassium are higher in plasma and serum, respectively), hence the same sample should be used if monitoring a patient sequentially. Both heparin and non-anticoagulant tubes can contain gels (also called separator tubes), which facilitate harvesting of serum (from the clot/cells) or heparinized plasma (from the cells). All of these tubes require centrifugation to separate serum (non-anticoagulant tubes) or plasma (anticoagulant tubes) from cells and should be handled similarly for optimal chemistry results (see above and below).
- Cytology: For fluid specimens (e.g. body cavity fluids), EDTA is preferred (preserves morphologic features). As indicated above, freshly made smears (with details provided regarding the type of smear, i.e. unconcentrated or direct or concentrated/centrifuged or sediment) should be provided with the EDTA. A non-anticoagulant tube can be used as well if culture is anticipated or if chemistry tests are warranted (e.g. measurement of bilirubin in a suspected bile duct rupture). If the fluid is hemorrhagic, collection into a red top will help distinguish between acute hemorrhage (or a splenic tap for abdominal fluid), which will clot, and pre-existing hemorrhage, which will not clot (blood defibrinates rapidly in body cavities).
Handling and storage
Samples should be handled appropriately after collection
- Separation: For hemostasis and chemistry testing, the plasma or serum should be separated from cells as soon as possible after sample collection, which is done by centrifugation. For serum, this involves allowing the sample to clot first and for serum to exude or separate from the clot. The latter can take a while in large animals (horses, ruminants), so rimming of the tube (inserting a wooden stick around the edges to separate the clot from the tube) may help facilitate clotting, which can take up to 30 minutes. With any tube, serum or plasma should be separated from cells and placed into a new clot (no anticoagulant) tube, which should labeled as “plasma” or “serum” as soon as possible after collection. Gel or serum/plasma separator tubes can be used (the gel facilitates separation of serum or plasma after centrifugation), but if they are old or damaged, the silicon may allow cellular constituents to leak through into the serum after centrifugation or may allow cells to metabolize glucose, therefore it is best if the serum or plasma is removed from the gel tube and placed into a new clot tube or another tube containing no anticoagulant.
- Labeling: All body fluid samples taken from a patient should be correctly labeled with the patient name or identification and the type of specimen (e.g. serum, plasma, synovial fluid, peritoneal fluid).
- Storage: All fluid samples should be stored at 4°C until submission. In contrast, slides should not be refrigerated (the cells lyse with storage).
Submission
- Label properly: Make sure all tubes and slides are labeled with patient identification and the contents (e.g. blood, urine).
- Package appropriately: Make sure the samples are protected from breakage. Cardboard slide boxes frequently break during transit, so if you are using them, wrapping them in bubble wrap or other cushioning material will protect them.
- Add cool packs: Ship fluid samples on cool packs. The samples should not be in direct contact with the cool pack, but wrapped in paper towels. Direct contact of cells with an ice pack will cause freezing of the sample and cell lysis.
- Avoid formalin: Do not ship formalin containers in the same package as the slides or tubes. Formalin readily leaks out of containers and affects the quality of the samples.
- Ship ASAP: The quicker the sample gets to the laboratory, the fewer the false changes in results due to storage.
- Provide a good history: This includes signalment (species, age, breed, sex of patient), history that may be relevant (e.g. travel, access to toxins, current medications), pertinent clinical signs (e.g. epistaxis) and, for cytology, a good description of the aspirated lesions (including imaging findings), e.g. multiple hypoechoic masses in the liver.
Related links
- Blood collection guidelines on this site:
- Blood collection guidelines (in greater detail) on the Animal Health Diagnostic Center website:
- Cytology collection guidelines on the Animal Health Diagnostic Center website:
- General information on cytologic specimens
- Collection and handling: Includes masses, body cavity fluids, urine, bone marrow samples.
